Description br> when submitting grades in scientific notation, a
Category:
Chemistry
-
Description
BR> When submitting grades in scientific notation, a number like 2300 would be 2.3e+3 or 0.0078 would be 7.8e-3.Remember entropy values are in units of joules.
must convert to kJ in some problems IF any errors pop up and do sometimes, please let me know!! -
Instructions
The sig fig checker has been turned off so feel free to enter near or exact numbers.
Assignment Submission
For this assignment, you submit answers by question parts. The number of submissions remaining for each question part only changes if you submit or change the answer.
Assignment Scoring
Your last submission is used for your score.
(b) Does the reaction lead to an increase or decrease in the disorder of the system?
(c)[Answer sig. to 0.1’s place] Calculate 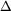 G° for the reaction at 298 K.
G° for the reaction at 298 K.
(b) 6 Cl2(g) + 2 Fe2O3(s) 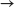 4 FeCl3(s) + 3 O2(g)
4 FeCl3(s) + 3 O2(g)
(c) 2 CH3OH(l) + 3 O2(g) 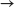 2 CO2(g) + 4 H2O(l)
2 CO2(g) + 4 H2O(l)


